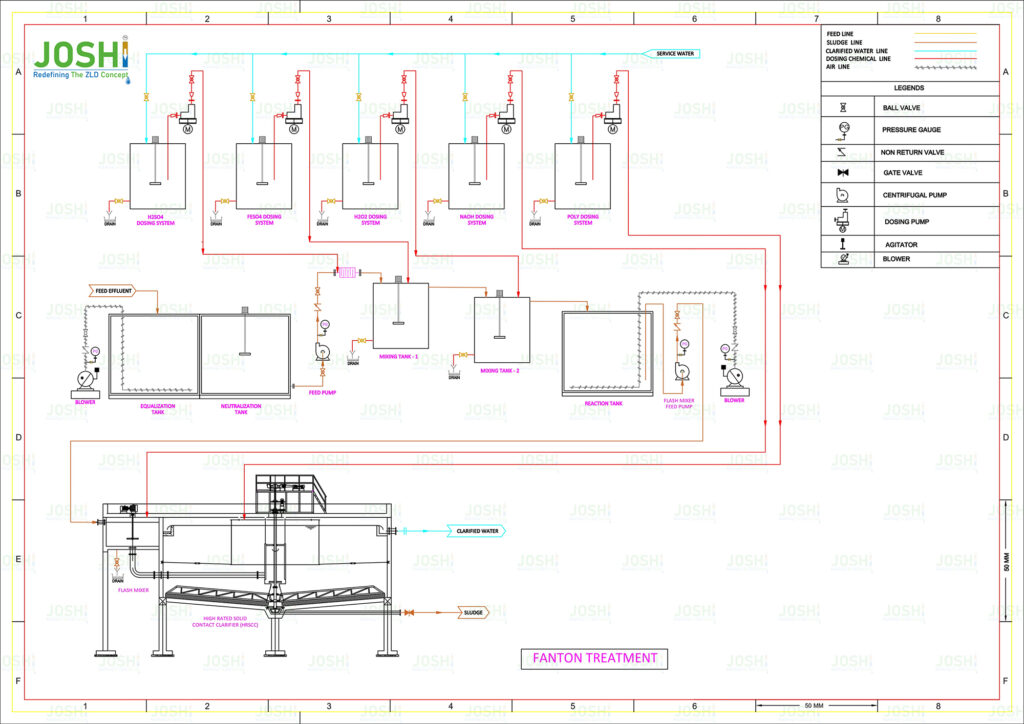
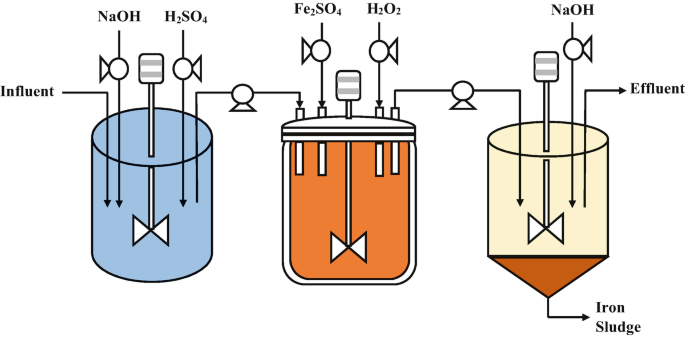
Fenton Process Reactor (FPR)
The Fenton process is an advanced oxidation process (AOP) used in wastewater treatment for the degradation of organic contaminants. It involves the use of a combination of hydrogen peroxide (H2O2) and a ferrous iron catalyst (typically ferrous sulfate, FeSO4) to generate highly reactive hydroxyl radicals (•OH). The Fenton process is typically used as a tertiary treatment step in wastewater treatment, following primary and secondary treatment processes.
System Components
In wastewater treatment, a Fenton process reactor is a vessel or system specifically design for the implementation of the Fenton process. It is the component where the Fenton reaction takes place and the oxidation of organic contaminants occurs.
A Fenton process reactor typically consists of the following key elements:
Reactor Vessel: The reactor vessel provides a controlled environment for the Fenton process to take place. It is typically made of corrosion-resistant materials such as stainless steel or glass-line steel to withstand the harsh chemical conditions.
Mixing System: A mixing system is employ to ensure the thorough mixing of the wastewater, hydrogen peroxide, and ferrous iron catalyst. This promotes the contact between the reactants and facilitates the Fenton reaction.
pH Control: The Fenton process is highly sensitive to pH conditions. The reactor may include a pH control system to maintain the optimal pH range for the Fenton reaction, which is typically between 2 and 4. Acid or alkali solutions can be added to adjust and control the pH as necessary.
Hydrogen Peroxide Dosage System: A hydrogen peroxide dosing system is employ to accurately control the addition of hydrogen peroxide to the reactor. This ensures the proper stoichiometric ratio with the ferrous iron catalyst for efficient hydroxyl radical generation.
Ferrous Iron Catalyst Dosage System: A ferrous iron catalyst dosing system is utilize to introduce the ferrous iron into the reactor. The ferrous iron is typically add as ferrous sulfate or another suitable iron salt, and its dosage is carefully controll to achieve the desire reaction kinetics.
Reaction Time: The wastewater, hydrogen peroxide, and ferrous iron catalyst remain in the reactor for a specific period to allow sufficient contact time for the Fenton reaction to occur. The reaction time is typically determine base on the specific wastewater characteristics and the desire level of oxidation.
Separation System: After the Fenton reaction, the treate wastewater needs to be separate from the reaction byproducts, including the precipitat iron sludge and other residual solids. Depending on the design, the reactor may incorporate settling tanks, clarifiers, or filtration systems for the separation of treate water from the solid residues.
The Fenton Process Steps:
Addition of Hydrogen Peroxide: Hydrogen peroxide is adde to the wastewater to provide a source of hydroxyl radicals. The concentration of hydrogen peroxide used depends on the target pollutant and the desire level of oxidation.
Addition of Ferrous Iron Catalyst: A ferrous iron catalyst, such as ferrous sulfate, is add to the wastewater. The ferrous iron acts as a catalyst, promoting the generation of hydroxyl radicals.
Reaction and Hydroxyl Radical Generation: In the presence of the ferrous iron catalyst, hydrogen peroxide undergoes a reaction called the Fenton reaction. This reaction leads to the formation of hydroxyl radicals through a series of chemical reactions, including the Haber-Weiss reaction and the iron-catalyzed decomposition of hydrogen peroxide:
Fe2+ + H2O2 → Fe3+ + •OH + OH-
Fe3+ + H2O2 → Fe2+ + •OH + H+
The hydroxyl radicals (•OH) generated in the Fenton process are highly reactive and can oxidize a wide range of organic contaminants, including persistent and recalcitrant compounds.
Contaminant Degradation: The hydroxyl radicals react with the organic contaminants in the wastewater, leading to the degradation of the pollutants. The hydroxyl radicals attack and break down the chemical bonds in the contaminants, transforming them into simpler and less harmful compounds, such as carbon dioxide (CO2), water (H2O), and other byproducts.
Significance & Advantages
Effective Oxidation: The hydroxyl radicals generated in the Fenton process have a high oxidation potential and can efficiently degrade a wide range of organic pollutants, including complex and persistent compounds.
Versatility: The Fenton process can be applied to various types of wastewater and is effective for the removal of various contaminants, including organic dyes, phenolic compounds, and recalcitrant organic pollutants.
Rapid Reaction: The Fenton process operates at relatively fast reaction rates, allowing for shorter treatment times compared to other oxidation processes.
Cost-effectiveness: The Fenton process utilizes readily available chemicals, such as hydrogen peroxide and ferrous iron catalysts, which are relatively low-cost compared to other advanced oxidation processes.
The design and configuration of a Fenton process reactor may vary depending on factors such as the wastewater flow rate, the target contaminants, and the treatment objectives. It is important to consider the appropriate reactor size, mixing intensity, dosing systems, and residence time to optimize the Fenton process performance and achieve effective oxidation of the contaminants.
Targeted Impurities
- Refractory COD
- Toxic COD
- Non-Biodigradable COD
- Recalcitrant Compounds